Key Takeaways
- COVID-19 is a virus illness that affects the lungs and breathing, causing
symptoms from mild cold-like signs to severe lung problems.
- Finding early signs of who might get very sick with COVID-19 can help doctors
provide better care sooner.
- Clinical trials offer access to emerging treatments and expert care that may not
be available elsewhere.
- Joining a COVID-19 trial can help advance research while potentially improving
your quality of life with supervised support.
A New Horizon in COVID-19 Research
Clinical trials are a cornerstone of medical advancement, offering the opportunity to test new treatments and
improve patient outcomes. This spotlight focuses on the Mi-RNA and COVID-19 trial, identified by the ID
NCT06881160. This study is actively exploring how certain molecules in the blood, called mi-RNAs, relate to
how severe COVID-19 can become and the risk of complications and death.
Understanding new research is a top priority for patients and caregivers seeking information on treatment
options, managing side effects, and exploring potential avenues for improvement. This post aims to provide a
clear overview of what this trial is about and how it could potentially impact the future of COVID-19 care.
Understanding COVID-19 and the Need for New Approaches
COVID-19 is a respiratory illness caused by the SARS-CoV-2 virus. It can cause symptoms ranging from mild to
severe lung failure. While some treatments and vaccines exist, researchers are continually working to better
predict who will become very sick and to develop better ways to manage the disease. Understanding the role of
mi-RNAs in COVID-19 may help improve the care and outcomes for patients.
Curated Facts and Expert Data
PubMed: This research compared miRNA expression patterns across different body samples like urine, blood,
and nose swabs from people with mild, moderate, and severe COVID-19. The study found that different illness
severities were linked to unique miRNA signatures, showing that miRNA testing from multiple body fluids
could help diagnose COVID-19 and predict how severe it will get.
[1].
Molecular Therapy (Cell Press): This study looked at small molecules called miRNAs in the blood of people
with COVID-19. It found that certain miRNAs, including hsa-miR-32-5p and hsa-miR-1246, can tell the
difference between those with no symptoms and people in critical condition. These miRNAs may work as
biomarkers, which means they help measure and identify how severe a case of COVID-19 is.
[2].
COVID-19 Symptoms
COVID-19 can manifest in various ways, but some of the most common symptoms include:
- Fever
- Cough
- Shortness of breath
- Fatigue
- Loss of taste or smell
- Chest pain
- Confusion
These symptoms can greatly affect daily life and health, especially when breathing is hard. Learning more
about these signs helps doctors and patients understand why some people get very sick and points to new
treatments like those studied in the Mi-RNA and COVID-19 trial.
Introducing the Trial's Focus: Diagnostic Test with mi-RNA
Analysis
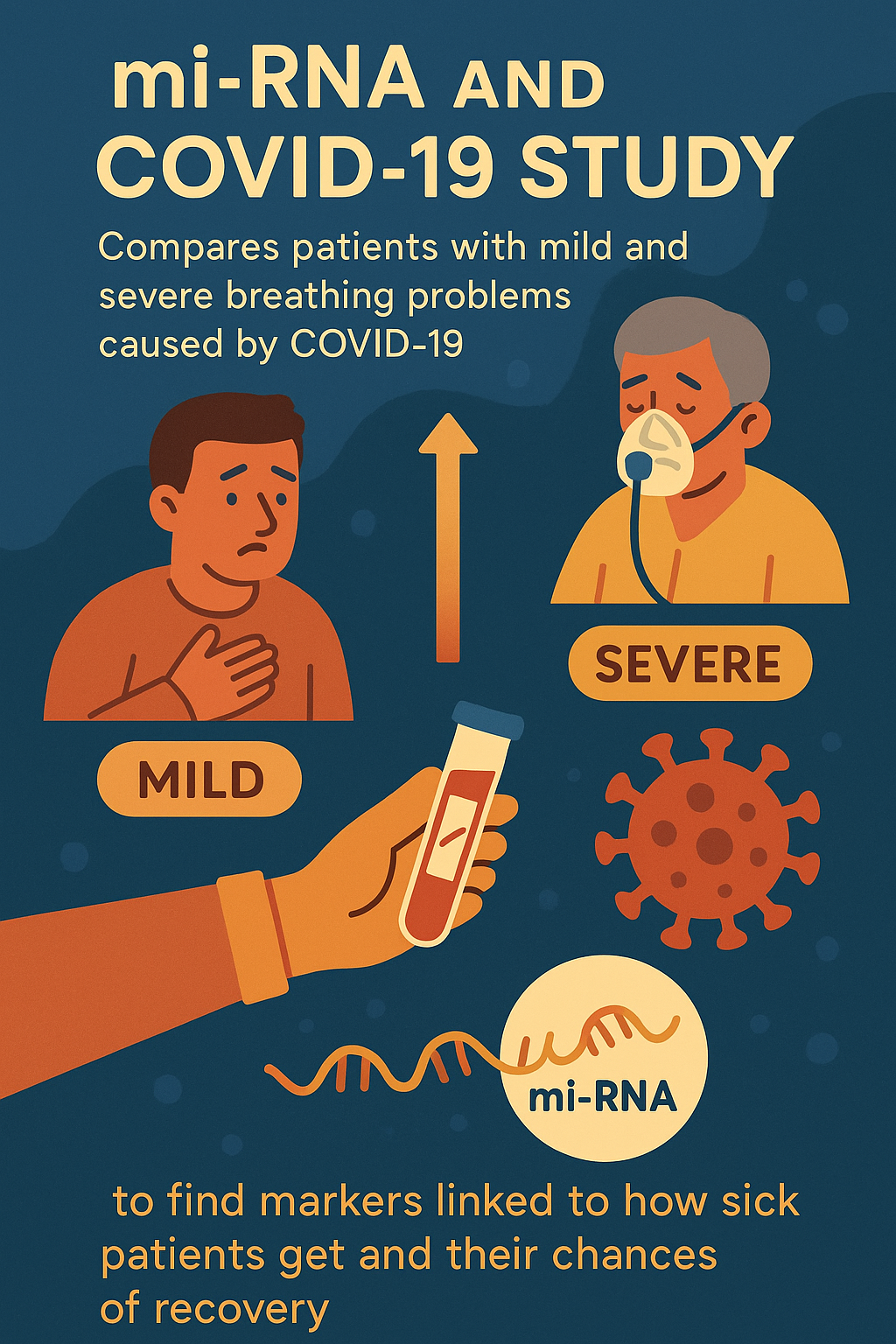
The Mi-RNA and COVID-19 trial is investigating a diagnostic test called mi-RNA analysis.
What is mi-RNA Analysis?
mi-RNA analysis looks at tiny molecules called microRNAs (mi-RNAs) in the blood. These molecules help control
how cells work and can show how the body responds to infection. By examining these mi-RNAs, doctors hope to
predict who might get sicker from COVID-19 and understand how the illness progresses.
This study is specifically designed to assess how mi-RNA levels relate to COVID-19 severity and outcomes,
helping find markers that could predict if a patient will have mild or severe respiratory problems.
Mechanism of Action
mi-RNAs are small pieces of genetic material that help control which genes are turned on or off in our cells.
Changes in mi-RNA levels during COVID-19 infection can influence how the virus affects the lungs and other
organs by changing the body's response to the infection.
This trial is unique because it aims to find blood markers (mi-RNAs) that can warn doctors early about
worsening COVID-19, which may help guide faster and more personalized treatments.
Trial design
This observational, case-control study compares patients with mild and severe respiratory failure caused by
COVID-19, analyzing their blood for mi-RNA differences to better understand disease progression.
Why is this Trial Important for Patients?
The Mi-RNA and COVID-19 trial embodies this crucial role in research, offering a potential path to:
-
Significantly improved outcomes for COVID-19: By evaluating mi-RNA patterns, this trial aims to find early
signs of worsening disease, potentially leading to faster care and better chances of recovery.
-
Developing more patient-friendly treatments: Finding mi-RNA markers might help doctors choose treatments
that fit each patient’s needs better, possibly reducing severe complications and hospital stays.
-
Advancing our understanding of COVID-19: The findings will increase knowledge about how COVID-19 affects the
body and how mi-RNAs play a role in disease severity, leading to future discoveries.
Who Can Participate? Eligibility at a Glance
Clinical trials have specific criteria to ensure the safety of participants and the validity of the study
results. For the Mi-RNA and COVID-19 trial, key eligibility criteria
include:
- Age: Participants must be 18 years or older
- Diagnosis: Confirmed SARS-CoV-2 infection by molecular testing on nose and throat swab
- Exclusion: Pregnant women are not eligible
What to Expect as a Participant
If you are deemed eligible and choose to enroll in the Mi-RNA and COVID-19 trial, you can expect the following
general process:
-
Screening: A series of tests and evaluations to confirm your eligibility at no cost.
-
Treatment Period: You will provide blood samples at the time of hospital admission for mi-RNA analysis.
There is no additional treatment given in this study.
-
Monitoring: Your health condition will be closely followed by the hospital team to track the severity of
COVID-19 and outcomes.
-
Follow-up: The study will review your disease outcome over an average of one year.
The duration of your participation will vary depending on the study design, but the research team will explain
the full commitment before enrollment.
Potential Risks and Benefits
Like all medical interventions, participation in a clinical trial carries both potential benefits and risks.
Potential Benefits:
- Access to a new treatment before it’s widely available.
- Close monitoring by a team of medical experts.
- Contributing to medical knowledge that could help future patients with COVID-19.
Potential Risks:
- The new treatment may not be effective for you.
- There is minimal risk as the study involves blood tests only, but blood drawing can cause minor discomfort
or bruising.
- The time commitment for study visits and procedures.
A detailed explanation of all potential risks and benefits will be provided during the informed consent
process.
How to Learn More and Get Involved
The Mi-RNA and COVID-19 trial (NCT06881160) is currently recruiting at Fondazione Policlinico Universitario
Agostino Gemelli IRCCS in Rome, Italy.
If you are interested in potentially participating, it is crucial to discuss these criteria with your
healthcare provider. They can determine if you meet the specific requirements. You may share this link with
your provider’s email address, or have them visit ClinQuestAi.com and type in NCT06881160.
You can also:
Remember: Deciding to participate in a clinical trial is a personal choice. It’s important to discuss all
aspects of the trial with your healthcare provider to ensure it’s the right decision for your individual
health situation.
Further Reading:
Our Commitment to Accuracy and Trust
All health-related content on this website, whether drafted by our team or with AI assistance, undergoes a
rigorous human review process before publication. Every article is fact-checked, edited, and medically
reviewed by a licensed medical professional with relevant expertise. Our goal is to provide information that
is accurate, aligned with current medical consensus, empathetic, and useful for patients and caregivers. We
are committed to transparency, clearly citing sources and displaying author and reviewer credentials to uphold
the highest standards of E-E-A-T (Experience, Expertise, Authoritativeness, and Trustworthiness).