Key Takeaways
- Influenza is a common flu virus infection that mostly affects the lungs and
breathing and can be serious especially for older adults.
- Getting flu vaccines is important because it can help prevent the flu or make
illness less severe if you do get sick.
- Clinical trials offer access to emerging treatments and expert care that may not
be available elsewhere.
- Joining a Influenza trial can help advance research while potentially improving
your quality of life with supervised support.
A New Horizon in Influenza Vaccine Research
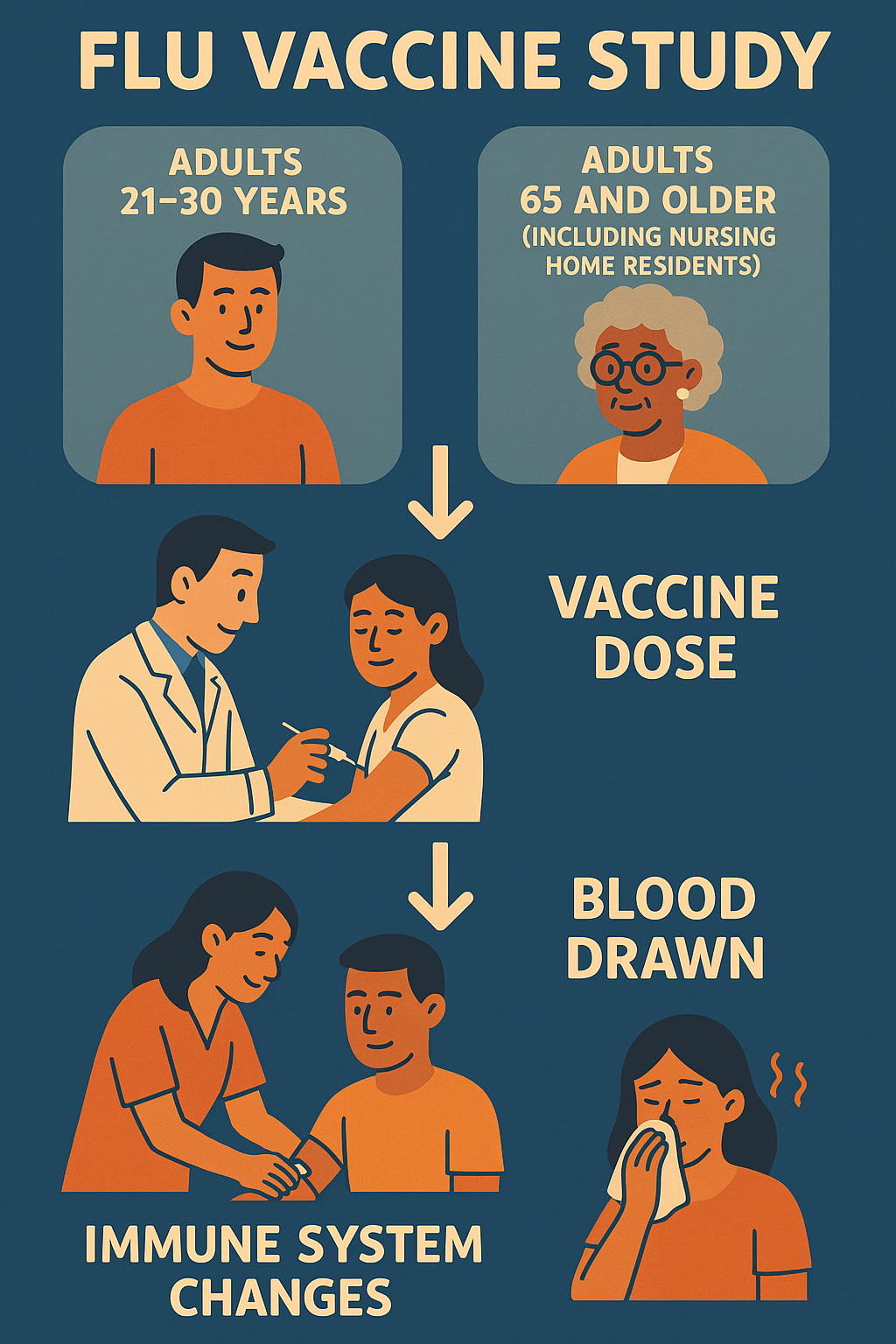
Clinical trials help create better ways to protect people from diseases. This study, called Systems
Investigation of Vaccine Responses in Aging and Frailty, looks at how two flu vaccines work in older adults,
especially those living in nursing homes who are more likely to get very sick from the flu.
Understanding new research is a top priority for patients and caregivers seeking information on treatment
options, managing side effects, and exploring potential avenues for improvement. This post aims to provide a
clear overview of what this trial is about and how it could potentially impact the future of Influenza care.
Understanding Influenza and Vaccine Needs
Influenza, often called the flu, is an infectious respiratory illness caused by flu viruses. It can cause
symptoms like fever, coughing, and body aches. Older adults and frail individuals are more vulnerable and can
face serious complications. This trial studies how two vaccines, Fluad and Fluzone, protect against the flu by
looking closely at immune responses to help find the best ways to protect these groups.
Curated Facts and Expert Data
PubMed: This study looked at how safe and effective two flu vaccines—Fluad (which has an added ingredient to
boost the immune response) and Fluzone (a standard flu shot)—are in 301 healthy adults. The results showed
that Fluad was mostly well tolerated, though people reported more pain where the shot was given and some
mild side effects. Fluad led to a stronger immune response than Fluzone, especially after the first shot.
[1].
PubMed: This Phase III clinical trial tested Fluad and Fluzone in 287 kids aged 6 months up to almost 6
years old in Mexico. It found that Fluad worked at least as well as Fluzone in helping the immune system
protect against all three flu strains and actually performed better overall. Both vaccines caused mostly
mild to moderate side effects and were considered safe for children.
[2].
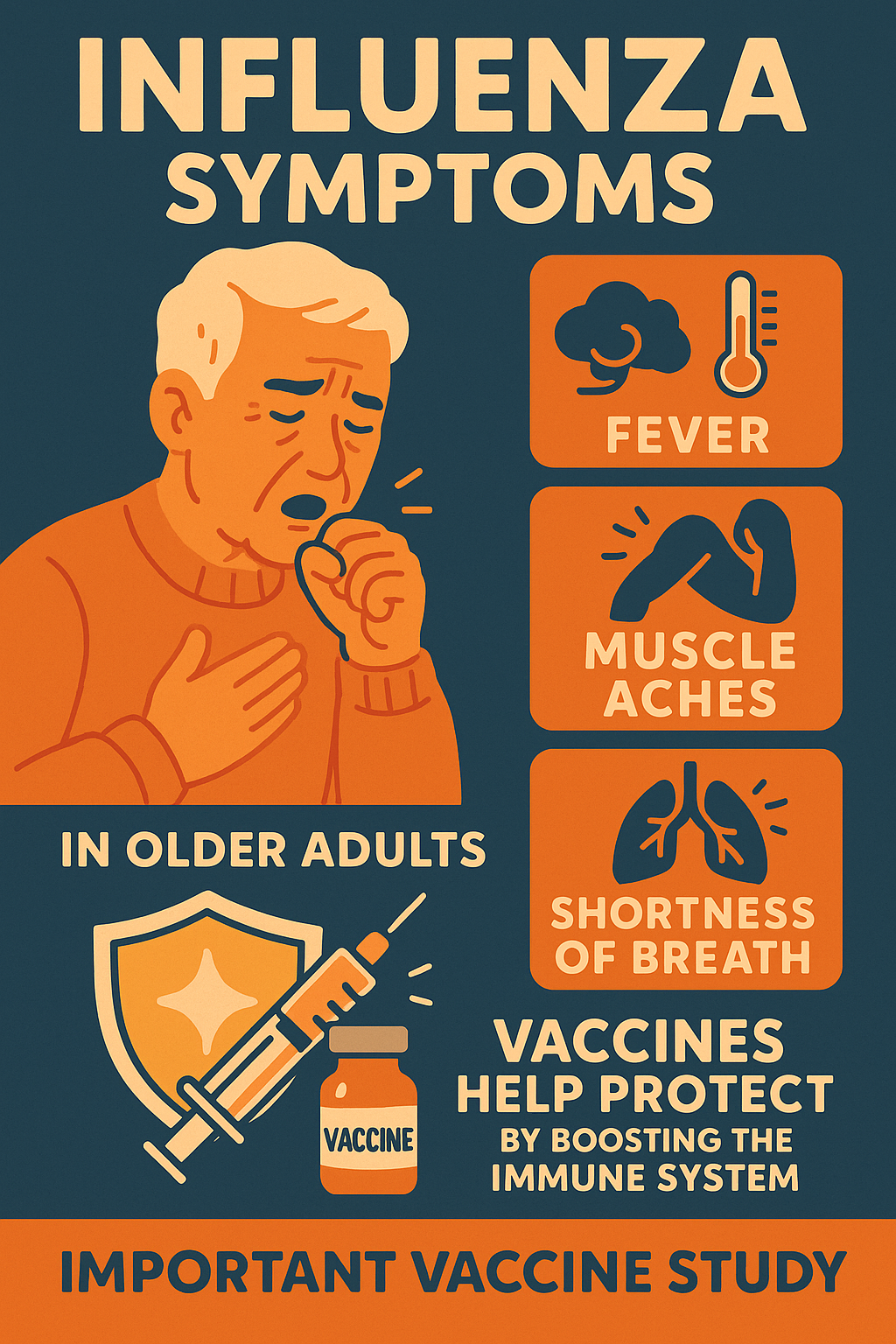
Influenza Symptoms
Influenza can manifest in various ways, but some of the most common symptoms include:
- Cough
- Sore throat
- Shortness of breath
- Nasal stuffiness
- Headache
- Malaise (general feeling of being unwell)
- Fever or feeling feverish
- Muscle aches
- Altered mental status (confusion or difficulty thinking clearly)
These symptoms can make daily activities difficult, especially for older adults. Understanding these helps
researchers improve vaccines like those studied in the Flu Vaccine Study.
Introducing the Trial's Focus: Comparing Fluad and Fluzone
Vaccines
The Flu Vaccine Study is testing two types of flu vaccines: Fluad, which has an added ingredient to boost the
immune system, and Fluzone High-Dose, which has more flu virus components to better protect older adults.
What are Fluad and Fluzone?
Fluad is a flu vaccine with MF59, a special ingredient that helps the body’s immune system respond stronger.
Fluzone High-Dose has four times the usual flu ingredient to help older people’s immune systems fight the flu
better.
This study is designed to see how safe and effective these two vaccines are in helping the immune system of
older adults and younger adults respond to the flu.
Mechanism of Action
Fluad’s MF59 ingredient acts like a helper to boost the body's immune response, making the vaccine work
better. Fluzone High-Dose delivers more of the flu virus parts to stimulate a stronger immune defense, aiming
to keep the flu away or lessen its severity.
Comparing the two vaccines directly in nursing home residents helps identify which vaccine induces a better
immune response in the people most vulnerable to the flu. This could lead to improved vaccination strategies
for older adults.
Trial design
The study randomly assigns participants to receive either vaccine without masking, ensuring fair comparison
while monitoring immune responses and symptoms following vaccination in both younger and older adults.
Why is this Trial Important for Patients?
The Flu Vaccine Study NCT05291676 embodies this crucial role in research, offering a potential path to:
-
Better outcomes for older adults facing influenza: By evaluating Fluad and Fluzone, the trial aims to
identify vaccines that provide stronger protection against the flu.
-
Developing vaccination approaches suited to nursing home residents who are at high risk for flu
complications.
-
Advancing scientific knowledge about immune responses to different flu vaccines, guiding future vaccine
development and use.
Who Can Participate? Eligibility at a Glance
Clinical trials have specific criteria to ensure the safety of participants and the validity of the study
results. For the Flu Vaccine Study trial, key eligibility criteria include:
- Age: Participants must be either 21-40 years or 65 years and older.
- Location: Must plan to stay in New Haven, CT area for 4-6 weeks during the study.
- Health Status: No acute infections, recent cancer treatment, or serious organ diseases like kidney failure
or HIV/AIDS.
- Consent: Must understand and give informed consent. For nursing home residents unable to consent,
surrogate consent plus verbal assent will be obtained.
- Other: Cannot be pregnant, have had serious adverse reactions to flu vaccines, recent blood donation, or
be currently in other clinical trials.
What to Expect as a Participant
If you join the Flu Vaccine Study, here’s what to expect:
-
Screening: A series of tests and evaluations to confirm your eligibility at no cost.
-
You will receive either the Fluad or Fluzone flu vaccine in one dose as part of the study, at no cost to
you.
-
Regular health check-ups and blood tests will be done to see how your immune system responds to the vaccines
and to watch for side effects.
-
Follow-up visits will happen for up to 70 days after vaccination to monitor health and immune responses.
The duration of your participation will vary depending on the study design, but the research team will explain
the full commitment before enrollment.
Potential Risks and Benefits
Like all medical interventions, participation in a clinical trial carries both potential benefits and risks.
Potential Benefits:
- Access to a new treatment before it’s widely available.
- Close monitoring by a team of medical experts.
- Contributing to medical knowledge that could help future patients with Influenza.
Potential Risks:
- The new treatment may not be effective for you.
- You might experience typical vaccine side effects like soreness or fever, and in rare cases, unexpected
reactions could occur. The study monitors safety closely.
- The time commitment for study visits and procedures.
A detailed explanation of all potential risks and benefits will be provided during the informed consent
process.
How to Learn More and Get Involved
The Flu Vaccine Study (NCT05291676) is currently recruiting at Yale School of Medicine in New Haven,
Connecticut, United States.
If you are interested in potentially participating, it is crucial to discuss these criteria with your
healthcare provider. They can determine if you meet the specific requirements. You may share this link with
your provider’s email address, or have them visit ClinQuestAi.com and type in NCT05291676.
You can also:
- Reach out the study coordinators: Irene Matos, RN, at
203-737-4739 or irene.matos@yale.edu for information.
Remember: Deciding to participate in a clinical trial is a personal choice. It’s important to discuss all
aspects of the trial with your healthcare provider to ensure it’s the right decision for your individual
health situation.
Further Reading:
Our Commitment to Accuracy and Trust
All health-related content on this website, whether drafted by our team or with AI assistance, undergoes a
rigorous human review process before publication. Every article is fact-checked, edited, and medically
reviewed by a licensed medical professional with relevant expertise. Our goal is to provide information that
is accurate, aligned with current medical consensus, empathetic, and useful for patients and caregivers. We
are committed to transparency, clearly citing sources and displaying author and reviewer credentials to uphold
the highest standards of E-E-A-T (Experience, Expertise, Authoritativeness, and Trustworthiness).