Key Takeaways
- Meningitis is an infection that affects the brain and spinal cord’s lining and
can cause fever, headache, and confusion.
- Finding better ways to detect meningitis early and predict how patients will
respond to treatment can save lives.
- Clinical trials offer access to emerging treatments and expert care that may not
be available elsewhere.
- Joining a Meningitis trial can help advance research while potentially improving
your quality of life with supervised support.
A New Horizon in Meningitis Research
Clinical trials are vital for improving medical care. This report highlights the Evaluation of the Levels of
Calcitonin Gene-related Peptide and Substance P trial (NCT06510751), which is studying how these peptides may
be used to improve diagnosis and treatment outcomes in patients with post-operative meningitis, a serious
infection that can occur after brain surgery.
Understanding new research is a top priority for patients and caregivers seeking information on treatment
options, managing side effects, and exploring potential avenues for improvement. This post aims to provide a
clear overview of what this trial is about and how it could potentially impact the future of Meningitis care.
Understanding Meningitis and the Need for New Approaches
Meningitis is an infection and inflammation of the protective membranes around the brain and spinal cord. It
is a serious neurological illness that can develop after surgery involving brain catheters, making diagnosis
challenging. Current tests may not always clearly identify the infection. Research like the Meningitis Peptide
Study is important to find new ways, such as checking neuropeptides like CGRP and substance P, to identify the
disease earlier and understand the treatment response better.
Curated Facts and Expert Data
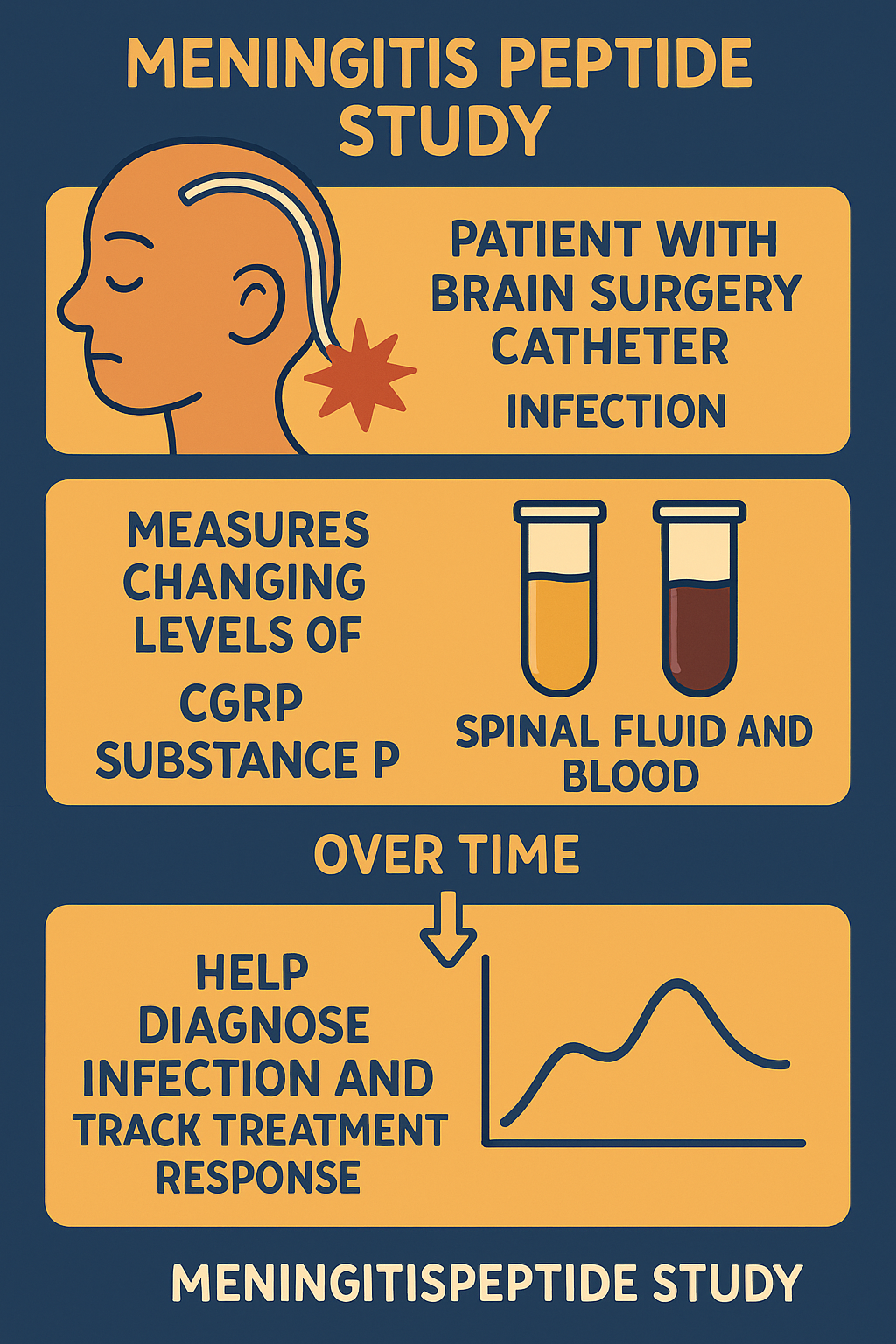
PubMed-indexed journal: This clinical trial measures the amounts of two molecules, CGRP and substance P, in
people who have had brain surgery and developed meningitis. The goal is to see if the levels of these
molecules in body fluids can help doctors better diagnose meningitis and monitor how patients respond to
treatment. The study focuses on infections related to the use of tubes placed in the brain, aiming to
improve early detection and outcome predictions.
[1].
PubMed-indexed journal: This scientific study looks at how a molecule called substance P affects blood
vessels in the brain during bacterial meningitis, using rats as a model. It shows that substance P causes
blood vessels on the brain’s surface to get wider during the early stages of meningitis. This widening of
blood vessels is one of the early body reactions to the infection.
[2].
Meningitis Symptoms
Meningitis can manifest in various ways, but some of the most common symptoms include:
- Fever
- Headache
- Confusion or trouble thinking clearly
- Stiff neck
- Sensitivity to light
- Nausea or vomiting
- Weakness or fatigue
These symptoms can make daily activities very hard for patients. Understanding and recognizing these symptoms
early helps patients get the right care quickly. The Meningitis Peptide Study aims to find better ways to
confirm the diagnosis and monitor how patients respond to treatment.
Introducing the Trial's Focus: Peptide Levels with CGRP and
Substance P
The Meningitis Peptide Study is observing how levels of two neuropeptides, called CGRP (calcitonin
gene-related peptide) and substance P, change in patients with post-operative meningitis.
What are CGRP and Substance P?
CGRP and substance P are tiny proteins called neuropeptides that nerves release, especially when there is
damage or inflammation. They can affect how the body reacts to infections like meningitis. This study measures
their amounts in blood and spinal fluid to see if they can help doctors diagnose meningitis or track how well
treatments are working.
This study is specifically designed to assess how useful CGRP and substance P levels are in diagnosing
meningitis caused by brain surgery and in predicting how patients respond to treatment.
Mechanism of Action
CGRP and substance P are neuropeptides that communicate signals in the nervous system, especially during
damage or infection. When meningitis occurs, these peptides may increase, influencing immune responses and
pain signals. Studying their levels helps scientists understand their role in disease and whether they can
serve as markers to guide treatment.
This trial is one of the few to analyze how CGRP and substance P change in patients with bacterial meningitis
after surgery. By using sensitive tests to measure these peptides in blood and spinal fluid, the study hopes
to find new markers that better detect meningitis and predict outcomes, potentially leading to faster, more
precise care.
Trial design
The study is designed as a prospective cohort observation, following patients suspected of meningitis in
intensive care to see how peptide levels correlate with disease progression and treatment success. This
approach helps gather real-world data without changing current treatments.
Why is this Trial Important for Patients?
The Meningitis Peptide Study (NCT06510751) embodies this crucial role in research, offering a potential path
to:
-
Significantly improved outcomes for meningitis: By evaluating CGRP and substance P, this trial aims to find
better ways to diagnose and manage meningitis, potentially leading to faster treatment and improved survival
rates.
-
Developing more patient-friendly diagnosis methods: Traditional tests can be slow or unclear. This study
explores if peptide levels can provide clearer, quicker information about infection and how patients respond
to antibiotics.
-
Advancing our understanding of meningitis: The findings will add valuable insight into how the nervous and
immune systems interact during infection, helping doctors and researchers improve care strategies.
Who Can Participate? Eligibility at a Glance
Clinical trials have specific criteria to ensure the safety of participants and the validity of the study
results. For the Meningitis Peptide Study trial, key eligibility criteria
include:
- Age: Participants must be 18 years or older
- Diagnosis: Patients with suspected meningitis related to cerebrospinal fluid drainage after brain surgery,
followed in intensive care
- Prior Treatment: Not specified; samples taken at routine clinical points before and during antibiotic
treatment
- General Health: Patients should be under intensive care for post-operative meningitis
- Other Factors: Must have undergone neurosurgical operation with cerebrospinal fluid drainage catheter
What to Expect as a Participant
If you are deemed eligible and choose to enroll in the Meningitis Peptide Study, you can expect the following
general process:
-
Screening: A series of tests and evaluations to confirm your eligibility at no cost.
-
Treatment Period: The study is observational, so you will receive standard care for meningitis. Researchers
will collect blood and spinal fluid samples during regular hospital tests at no cost.
-
Monitoring: Your health status, including consciousness level, fever, organ function, and lab tests, will be
recorded regularly to understand how the disease progresses and responds to treatment.
-
Follow-up: Data related to your intensive care stay and outcomes, such as survival, will be monitored and
analyzed over the study period, lasting up to about 16 months.
The duration of your participation will vary depending on the study design, but the research team will explain
the full commitment before enrollment.
Potential Risks and Benefits
Like all medical interventions, participation in a clinical trial carries both potential benefits and risks.
Potential Benefits:
- Access to a new treatment before it’s widely available.
- Close monitoring by a team of medical experts.
- Contributing to medical knowledge that could help future patients with Meningitis.
Potential Risks:
- The new treatment may not be effective for you.
- Since this is an observational study collecting samples during routine care, risks are minimal. However,
as with all infections, meningitis can have serious complications.
- The time commitment for study visits and procedures.
A detailed explanation of all potential risks and benefits will be provided during the informed consent
process.
How to Learn More and Get Involved
The Meningitis Peptide Study (NCT06510751) is currently recruiting at Akdeniz University School of Medicine,
Department of Anesthesiology and Intensive Care in Antalya, Turkey.
If you are interested in potentially participating, it is crucial to discuss these criteria with your
healthcare provider. They can determine if you meet the specific requirements. You may share this link with
your provider’s email address, or have them visit ClinQuestAi.com and type in NCT06510751.
You can also:
- Contact the study coordinators at +90 242 249 6231 for more
information.
Remember: Deciding to participate in a clinical trial is a personal choice. It’s important to discuss all
aspects of the trial with your healthcare provider to ensure it’s the right decision for your individual
health situation.
Further Reading:
Our Commitment to Accuracy and Trust
All health-related content on this website, whether drafted by our team or with AI assistance, undergoes a
rigorous human review process before publication. Every article is fact-checked, edited, and medically
reviewed by a licensed medical professional with relevant expertise. Our goal is to provide information that
is accurate, aligned with current medical consensus, empathetic, and useful for patients and caregivers. We
are committed to transparency, clearly citing sources and displaying author and reviewer credentials to uphold
the highest standards of E-E-A-T (Experience, Expertise, Authoritativeness, and Trustworthiness).