Key Takeaways
- Rare diseases like Long COVID, pancreatitis, and sarcoidosis affect people's
daily lives with symptoms like fatigue and breathing problems.
- Using new technology like apps and wearable devices can help people better track
their symptoms and manage their health.
- Clinical trials offer access to emerging treatments and expert care that may not
be available elsewhere.
- Joining a Rare Diseases including Long COVID, Pancreatitis, Sarcoidosis, VCP
disease, Primary Ciliary Disease trial can help advance research while potentially improving your quality of
life with supervised support.
A New Horizon in Rare Disease Research
Clinical trials are key to medical advancement, providing opportunities to test new tools and treatments to
improve patient care. The CZI Rare As One trial (NCT06907953) is exploring innovative ways to help people with
rare diseases track and manage their symptoms using apps and wearables, aiming to support better health
outcomes.
Understanding new research is a top priority for patients and caregivers seeking information on treatment
options, managing side effects, and exploring potential avenues for improvement. This post aims to provide a
clear overview of what this trial is about and how it could potentially impact the future of Rare Diseases
including Long COVID, Pancreatitis, Sarcoidosis, VCP disease, Primary Ciliary Disease care.
Understanding Rare Diseases and the Need for Better Symptom
Tracking
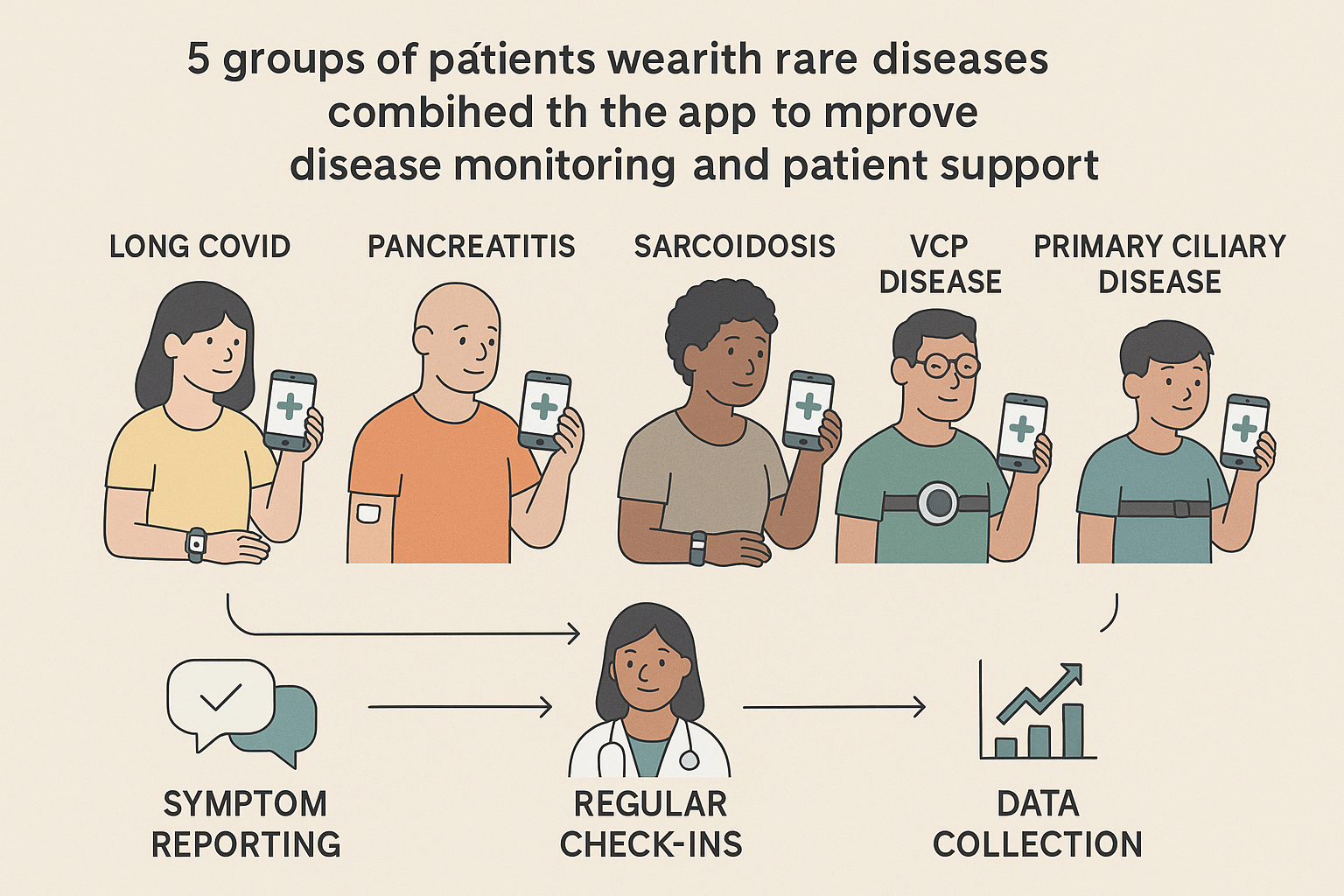
Rare diseases like Long COVID, chronic pancreatitis, sarcoidosis, VCP disease, and primary ciliary disease
affect small groups of people with various symptoms such as fatigue, breathing difficulties, and pain. Because
these conditions often have complex symptoms, new tools that help people monitor their health daily are
important. The Rare Disease App Study aims to develop such tools to help people and their doctors better
understand and manage these diseases.
Curated Facts and Expert Data
trialstoday.org: This study is funded by the Chan Zuckerberg Initiative and works directly with patients and
caregivers to design and test a digital app and wearable device system. The focus is on tracking symptoms
for rare diseases, including Long COVID, Pancreatitis, Sarcoidosis, VCP disease, and Primary Ciliary
Disease. Patients use these digital tools to keep daily records of their symptoms, which helps both them and
researchers see how their illness changes over time. This co-designed approach allows people to actively and
passively monitor their health in real-time.
[1].
arxiv.org: This study tests a new way to measure how severe Long COVID and Chronic Fatigue Syndrome are
using a wearable sensor placed on the ankle. Participants wore the device for a week, and researchers
tracked their "UpTime," a number showing how active they are during the day. Results showed that this method
could clearly separate healthy people from those with these rare diseases, making it a useful way to measure
symptoms objectively.
[2].
Common Symptoms of Included Rare Diseases
Rare Diseases including Long COVID, Pancreatitis, Sarcoidosis, VCP disease, Primary Ciliary Disease can
manifest in various ways, but some of the most common symptoms include:
- Fatigue
- Shortness of breath
- Cough
- Memory changes
- Headache
- Lightheadedness
- Bloating
- Constipation
- Diarrhea
- Pain
- Sleep disturbance
These symptoms can make daily activities hard and reduce quality of life. Tracking them with new technologies
can provide helpful information for patients and health providers.
Introducing the Study's Focus: Symptom Tracking Using Apps
and Wearable Devices
The Rare Disease App Study is testing a smartphone app combined with wearable gadgets to help people with rare
diseases record and send information about their symptoms easily every day.
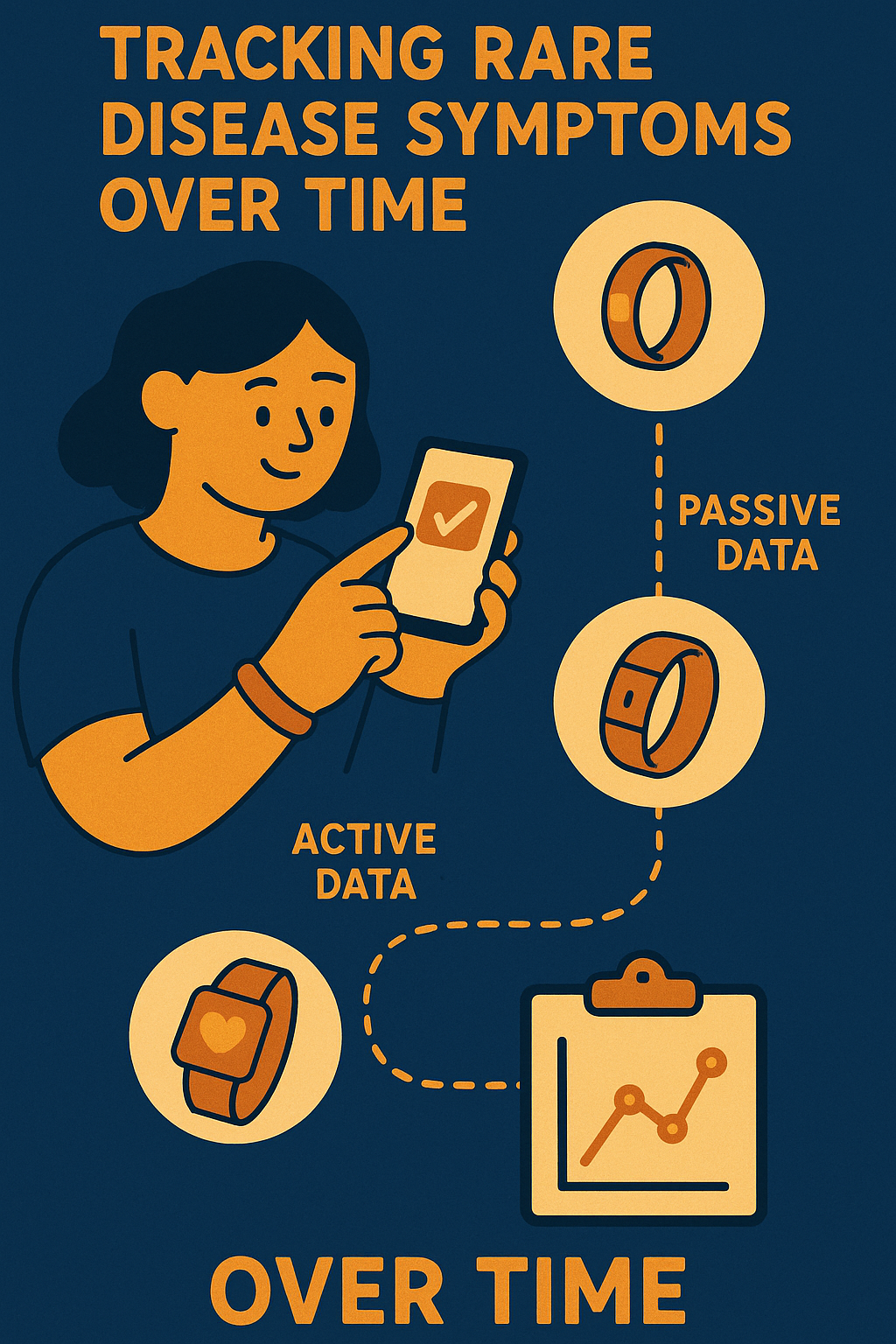
What Are Apps and Wearables?
Apps are programs you use on your phone, designed here to let you report how you're feeling and track symptoms
over time. Wearable devices like smart rings, wristbands, and watches can collect health data like heart rate
and sleep patterns without needing you to do anything extra. Together, they help keep a clear picture of
health.
This study aims to check how well these new tools work for patients, including how easy they are to use and if
people stick with using them over several months.
Mechanism of Action
Wearable devices collect health signals like heart rate, sleep quality, and breathing patterns passively,
while the app lets patients actively report symptoms and daily routines through simple tasks and surveys. This
combination offers a fuller picture of health and disease impact.
This study combines patient co-designed technology with wearables to improve symptom tracking in rare
diseases, aiming for tools that are easy to use and provide valuable health information without adding burden
on patients.
Trial design
This is an observational, prospective cohort study where participants use the app and wearables over time to
provide feedback and real-life data to test usability, adherence, and benefits.
Why is this Trial Important for Patients?
The Rare Disease App Study (NCT06907953) embodies this important research by:
-
Helping people with rare diseases better track symptoms daily to improve disease understanding and personal
health management.
-
Creating patient-friendly digital health tools that encourage long-term use without interfering with daily
life.
-
Generating insights that could guide future development of supportive technologies and care strategies for
rare diseases.
Who Can Participate? Eligibility at a Glance
Clinical trials have specific criteria to ensure the safety of participants and the validity of the study
results. For the Rare Disease App Study trial, key eligibility criteria
include:
- Age: Participants must be 18 years or older, except for primary ciliary disease (PCD) group where age 14+
is allowed
- Diagnosis: Must have a clinician-confirmed or suspected diagnosis of Long COVID, chronic pancreatitis,
sarcoidosis, VCP disease, or primary ciliary disease (PCD)
- Mobile Device: Must own and use a personal smartphone compatible with the study app (iPhone 6s/iOS15+ or
iPhone 8/iOS16+ or Android version 12+ depending on subgroup)
- Residence: Must live in the United States
- Willingness: Must be willing to use wearable devices provided for the study
What to Expect as a Participant
If you join the Rare Disease App Study, here is what you can expect:
-
Screening: A series of tests and evaluations to confirm your eligibility at no cost.
-
Treatment Period: Use the study app and wearable devices like smart rings or watches daily for about 5
months to track symptoms and health data at no cost to you.
-
Monitoring: Study staff will call you every two weeks to provide support, answer questions, solve technical
problems, and gather your feedback.
-
Follow-up: You will continue to send symptom reports and data through the app during the study period to
help researchers understand usability and benefits.
The duration of your participation will vary depending on the study design, but the research team will explain
the full commitment before enrollment.
Potential Risks and Benefits
Like all medical interventions, participation in a clinical trial carries both potential benefits and risks.
Potential Benefits:
- Access to a new treatment before it’s widely available.
- Close monitoring by a team of medical experts.
- Contributing to medical knowledge that could help future patients with Rare Diseases including Long COVID,
Pancreatitis, Sarcoidosis, VCP disease, Primary Ciliary Disease.
Potential Risks:
- The new treatment may not be effective for you.
- There are minimal risks as this is an observational study using devices and reporting symptoms, but
participants may experience inconvenience or privacy concerns related to technology use.
- The time commitment for study visits and procedures.
A detailed explanation of all potential risks and benefits will be provided during the informed consent
process.
How to Learn More and Get Involved
The Rare Disease App Study (NCT06907953) is currently recruiting participants in New York, United States, and
is open at various sites across the US.
If you are interested in potentially participating, it is crucial to discuss these criteria with your
healthcare provider. They can determine if you meet the specific requirements. You may share this link with
your provider’s email address, or have them visit ClinQuestAi.com and type in NCT06907953.
You can also:
- Contact the study coordinators at friend@4youandme.org or
sarah@4youandme.org for more information about joining.
Remember: Deciding to participate in a clinical trial is a personal choice. It’s important to discuss all
aspects of the trial with your healthcare provider to ensure it’s the right decision for your individual
health situation.
Further Reading:
Our Commitment to Accuracy and Trust
All health-related content on this website, whether drafted by our team or with AI assistance, undergoes a
rigorous human review process before publication. Every article is fact-checked, edited, and medically
reviewed by a licensed medical professional with relevant expertise. Our goal is to provide information that
is accurate, aligned with current medical consensus, empathetic, and useful for patients and caregivers. We
are committed to transparency, clearly citing sources and displaying author and reviewer credentials to uphold
the highest standards of E-E-A-T (Experience, Expertise, Authoritativeness, and Trustworthiness).